Compliant Content Excellence
How to Build Pharma Commercial Content Workflows
Pharmaceutical companies face a dual imperative: accelerate content creation while upholding uncompromising regulatory standards. With content production surging 29% but 77% of approved assets never used, achieving operational excellence in compliant workflows is no longer a luxury, it’s a competitive differentiator.
Content operational excellence ensures every asset is accurate, timely, and resource-efficient. It delivers measurable ROI by minimizing waste and maximizing value, serving both the organization and the end user. Patients and providers receive the right information at the right moment in their learning journey, whether it involves diagnostics, available therapies, or ongoing care.
At the core of this excellence lie three foundational principles that define every high-performing pharma content operation:
Align Resources & Incentives
Ensure teams are guided by a unified brand vision, aligned objectives, and clearly defined success metrics that connect effort to impact.
Build Trust & Accountability
Strengthen cross-functional collaboration by valuing diverse expertise, fostering transparency, and creating a shared operational language.
Balance Risk & Creativity
Drive innovation responsibly, encouraging bold ideas while maintaining unwavering adherence to regulatory and compliance standards.
This guide outlines ten steps to build an efficient, compliant, and scalable pharma content ecosystem, one grounded in these core principles and designed to equip teams with world-class operational foundations.
10-Step Implementation Plan
Step 1: Research & Discovery
Audit your current workflow to establish a clear baseline. Map content origins, cross-functional handoffs, review timelines, and bottlenecks. Identify where compliance gaps and inefficiencies occur.
Step 2: Prioritize Pain Points and Diagnose Root Causes
Identify the top three workflow pain points that create delays or risk. Analyze underlying causes; often cultural misalignment, unclear ownership, or MLR uncertainty. Engage stakeholders directly to uncover challenges that process maps alone can’t reveal.
Step 3: Establish a Steering Committee & Governance Framework
Form a cross-functional governance group (Medical, Legal, Regulatory, Brand, AI Compliance) to own SOP development. This will help ensure that content collaborators trust each other in their professional capacity and in their professional judgment. Define clear roles, approval authority, escalation paths, and accountability mechanisms. Anchor all activity to a centralized content hub that serves as the single source of truth.
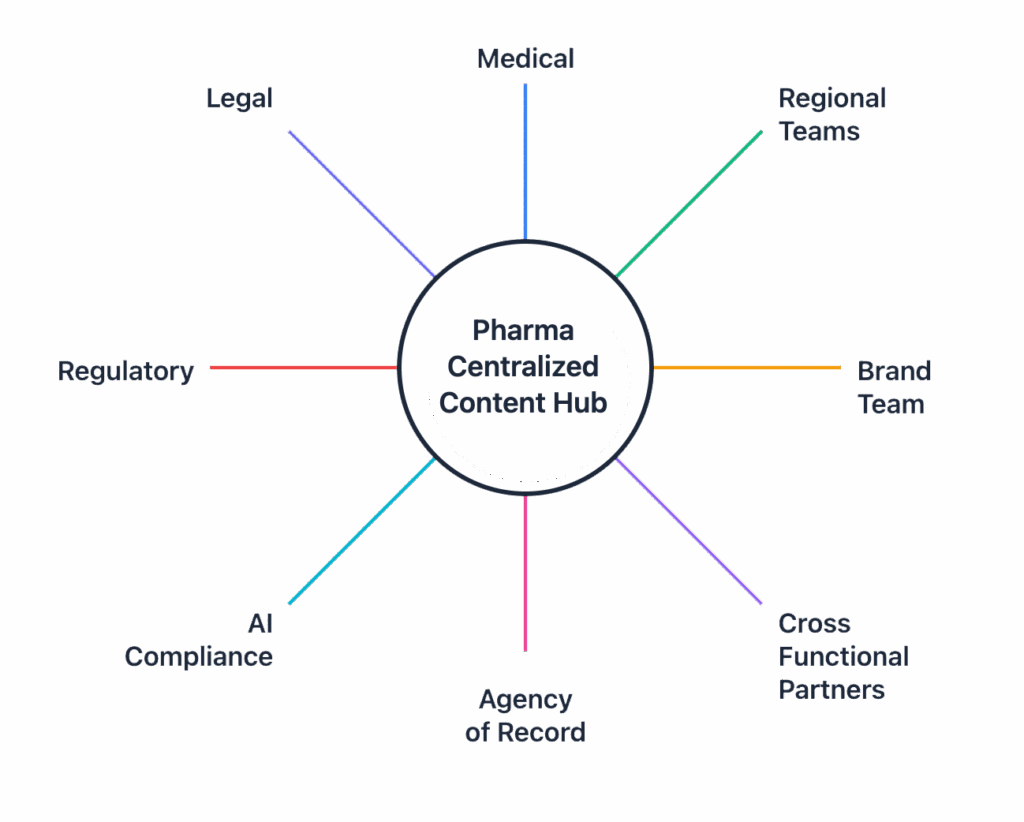
Step 4: Build a Centralized Content Hub
Create an integrated system to store, tag, and version all approved materials that is available to all stakeholders.. Enable modular reuse, real-time visibility, and audit-ready documentation.
Step 5: Develop User-Centric SOPs & Workflows
Design accessible, visual SOPs and templates covering:
– Content creation standards
– Review and approval workflows
– Quality assurance and risk management
– Stakeholder distribution and deployment protocols
– Metadata and content tagging
– Translation protocols and standards
– AI-use governance and compliance safeguards
Use visual flowcharts, plain language, and version control to ensure usability and adoption.
Step 6: Establish Meeting & Communication Cadence
Establish feedback templates, approval forms, and clear escalation pathways. Schedule consistent cross-functional meetings between the hub and stakeholder teams to maintain alignment and resolve issues quickly.
Step 7: Drive Content Through Campaign Execution
Oversee end-to-end workflows across internal teams and Agency of Record. Coordinate global markets, manage legal/regulatory reviews, and track timelines and approvals.
Step 8: Operationalize Data & Insights Monitoring
Set up dashboards to track performance metrics, turnaround times, approval rates, and content utilization. Use insights to continuously refine workflow efficiency and enforce operational excellence.
Step 9: Accelerate Creativity and Efficiency Gains with AI Training
Train teams on responsible AI usage, including prompt design, output interpretation, and compliance guardrails. Demonstrate how AI can fast-track modular content creation, streamline compliance checks, and reduce review cycles.
It takes a lot of effort to build, test, and pilot these AI tools in content workflows on top of everybody’s day-to-day work. In order for us to be able to start seeing the benefits, organizations need to provide general education on how AI works to empower their employees to develop more agency.
Step 10: Maintain Awareness and Buy-In with Educational Internal Communications
Launch an internal newsletter to share issuance of regulatory guidelines on the use of AI from agencies like the US Food and Drug Administration and European Medicines Agency, protocol updates, and success stories. Track engagement metrics to identify knowledge gaps and reinforce best practices organization-wide.
Interested in elevating your pharma content operations?
Schedule a call with Jill to learn how to implement a compliant content excellence framework tailored to your pharma organization.



